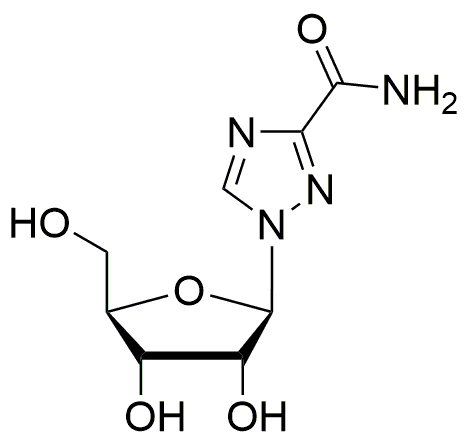
Ribavirin is widely utilized in research focused on:
- Antiviral Treatments: Primarily used in combination therapies for treating viral infections such as hepatitis C and respiratory syncytial virus (RSV), providing a broader spectrum of antiviral activity.
- Research on Viral Pathogenesis: Serves as a critical tool in laboratories studying the mechanisms of viral replication and pathogenesis, helping researchers develop new antiviral strategies.
- Pharmaceutical Development: Integral in the formulation of antiviral drugs, allowing pharmaceutical companies to create effective treatments with improved efficacy and safety profiles.
- Clinical Trials: Frequently employed in clinical research to evaluate the effectiveness of new antiviral therapies, contributing to advancements in infectious disease management.
- Biotechnology Applications: Used in the development of vaccines and therapeutic agents, enhancing the ability to combat emerging viral threats and improve public health outcomes.
General Information
Properties
Safety and Regulations
Applications
Ribavirin is widely utilized in research focused on:
- Antiviral Treatments: Primarily used in combination therapies for treating viral infections such as hepatitis C and respiratory syncytial virus (RSV), providing a broader spectrum of antiviral activity.
- Research on Viral Pathogenesis: Serves as a critical tool in laboratories studying the mechanisms of viral replication and pathogenesis, helping researchers develop new antiviral strategies.
- Pharmaceutical Development: Integral in the formulation of antiviral drugs, allowing pharmaceutical companies to create effective treatments with improved efficacy and safety profiles.
- Clinical Trials: Frequently employed in clinical research to evaluate the effectiveness of new antiviral therapies, contributing to advancements in infectious disease management.
- Biotechnology Applications: Used in the development of vaccines and therapeutic agents, enhancing the ability to combat emerging viral threats and improve public health outcomes.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Número de catálogo
Número de lote/lote
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.