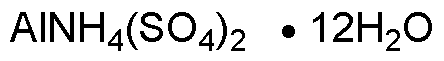Ammonium aluminum sulfate dodecahydrate is widely utilized in research focused on:
- Water Purification: This compound is commonly used as a coagulant in water treatment processes, helping to remove impurities and improve water clarity.
- Food Industry: It serves as a food additive, particularly in baking powder, where it acts as a leavening agent, helping baked goods rise and achieve a light texture.
- Textile Industry: In dyeing processes, it acts as a mordant, enhancing the adherence of dyes to fabrics, which results in brighter and more durable colors.
- Cosmetics: It is used in various personal care products for its astringent properties, helping to tighten skin and reduce oiliness.
- Laboratory Reagents: Researchers utilize it in analytical chemistry as a reagent for various experiments, particularly in the determination of certain metal ions.
General Information
Properties
Safety and Regulations
Applications
Ammonium aluminum sulfate dodecahydrate is widely utilized in research focused on:
- Water Purification: This compound is commonly used as a coagulant in water treatment processes, helping to remove impurities and improve water clarity.
- Food Industry: It serves as a food additive, particularly in baking powder, where it acts as a leavening agent, helping baked goods rise and achieve a light texture.
- Textile Industry: In dyeing processes, it acts as a mordant, enhancing the adherence of dyes to fabrics, which results in brighter and more durable colors.
- Cosmetics: It is used in various personal care products for its astringent properties, helping to tighten skin and reduce oiliness.
- Laboratory Reagents: Researchers utilize it in analytical chemistry as a reagent for various experiments, particularly in the determination of certain metal ions.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Número de catálogo
Número de lote/lote
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.