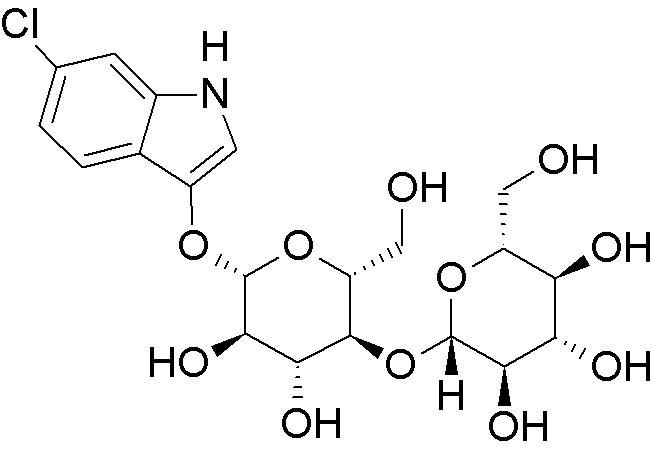
6-Chloro-3-indoxyl-b-D-cellobioside is widely utilized in research focused on:
- Plant Biotechnology: This compound serves as a substrate for detecting gene expression in transgenic plants, allowing researchers to visualize the activity of specific genes through colorimetric assays.
- Enzyme Activity Studies: It acts as a substrate for various glycosyltransferases, helping scientists study enzyme kinetics and mechanisms, which is crucial for understanding metabolic pathways.
- Pharmaceutical Research: The compound is used in drug development processes, particularly in screening for potential therapeutic agents that target specific biological pathways.
- Microbial Studies: It aids in identifying and characterizing microbial enzymes, providing insights into microbial metabolism and potential biotechnological applications.
- Analytical Chemistry: This chemical is utilized in the development of analytical methods for detecting and quantifying glycosylated compounds, enhancing the accuracy of biochemical assays.
General Information
Properties
Safety and Regulations
Applications
6-Chloro-3-indoxyl-b-D-cellobioside is widely utilized in research focused on:
- Plant Biotechnology: This compound serves as a substrate for detecting gene expression in transgenic plants, allowing researchers to visualize the activity of specific genes through colorimetric assays.
- Enzyme Activity Studies: It acts as a substrate for various glycosyltransferases, helping scientists study enzyme kinetics and mechanisms, which is crucial for understanding metabolic pathways.
- Pharmaceutical Research: The compound is used in drug development processes, particularly in screening for potential therapeutic agents that target specific biological pathways.
- Microbial Studies: It aids in identifying and characterizing microbial enzymes, providing insights into microbial metabolism and potential biotechnological applications.
- Analytical Chemistry: This chemical is utilized in the development of analytical methods for detecting and quantifying glycosylated compounds, enhancing the accuracy of biochemical assays.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Numéro de catalogue
Numéro de lot/série
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.