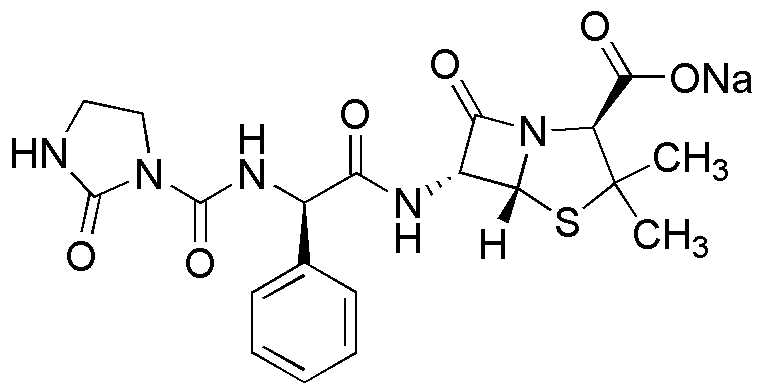
Azlocillin sodium salt is widely utilized in research focused on
- Antibiotic Development: This compound is primarily used in the development of broad-spectrum antibiotics, particularly for treating infections caused by Gram-negative bacteria. Its effectiveness in combating resistant strains makes it a valuable asset in pharmaceutical research.
- Pharmaceutical Formulations: Azlocillin sodium salt is incorporated into various injectable formulations, enhancing the efficacy of treatments for serious infections. Its solubility and stability in solution are advantageous for creating reliable medications.
- Clinical Trials: Researchers often use this compound in clinical trials to evaluate its safety and effectiveness in treating conditions like pneumonia and urinary tract infections, providing critical data for regulatory approvals.
- Combination Therapies: It is frequently studied in combination with other antibiotics to enhance therapeutic outcomes, particularly in patients with polymicrobial infections, offering a synergistic effect that can improve patient recovery rates.
- Research on Resistance Mechanisms: Azlocillin sodium salt is also employed in studies aimed at understanding bacterial resistance mechanisms, helping scientists develop new strategies to overcome antibiotic resistance, a growing global health concern.
General Information
Properties
Safety and Regulations
Applications
Azlocillin sodium salt is widely utilized in research focused on
- Antibiotic Development: This compound is primarily used in the development of broad-spectrum antibiotics, particularly for treating infections caused by Gram-negative bacteria. Its effectiveness in combating resistant strains makes it a valuable asset in pharmaceutical research.
- Pharmaceutical Formulations: Azlocillin sodium salt is incorporated into various injectable formulations, enhancing the efficacy of treatments for serious infections. Its solubility and stability in solution are advantageous for creating reliable medications.
- Clinical Trials: Researchers often use this compound in clinical trials to evaluate its safety and effectiveness in treating conditions like pneumonia and urinary tract infections, providing critical data for regulatory approvals.
- Combination Therapies: It is frequently studied in combination with other antibiotics to enhance therapeutic outcomes, particularly in patients with polymicrobial infections, offering a synergistic effect that can improve patient recovery rates.
- Research on Resistance Mechanisms: Azlocillin sodium salt is also employed in studies aimed at understanding bacterial resistance mechanisms, helping scientists develop new strategies to overcome antibiotic resistance, a growing global health concern.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.