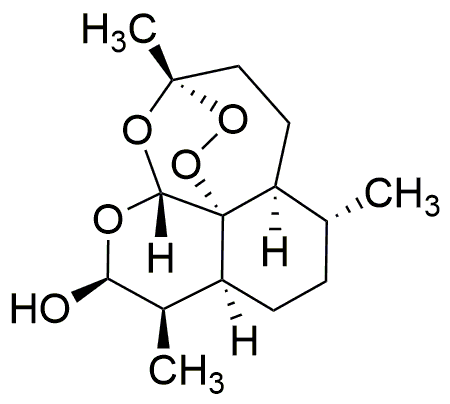
Dihydroartemisinin is widely utilized in research focused on:
- Antimalarial Treatments: This compound is a key ingredient in artemisinin-based combination therapies (ACTs), which are the standard treatment for malaria. Its effectiveness against resistant strains makes it invaluable in public health.
- Cancer Research: Studies have shown that dihydroartemisinin exhibits anticancer properties, potentially inhibiting the growth of various cancer cell lines. This opens avenues for developing new cancer therapies.
- Anti-inflammatory Applications: The compound has demonstrated anti-inflammatory effects, making it a candidate for treating conditions like arthritis and other inflammatory diseases.
- Drug Delivery Systems: Dihydroartemisinin can be incorporated into nanoparticles for targeted drug delivery, enhancing the efficacy and reducing side effects of treatments.
- Veterinary Medicine: Its use extends to veterinary applications, particularly in treating parasitic infections in livestock, contributing to better animal health and productivity.
General Information
Properties
Safety and Regulations
Applications
Dihydroartemisinin is widely utilized in research focused on:
- Antimalarial Treatments: This compound is a key ingredient in artemisinin-based combination therapies (ACTs), which are the standard treatment for malaria. Its effectiveness against resistant strains makes it invaluable in public health.
- Cancer Research: Studies have shown that dihydroartemisinin exhibits anticancer properties, potentially inhibiting the growth of various cancer cell lines. This opens avenues for developing new cancer therapies.
- Anti-inflammatory Applications: The compound has demonstrated anti-inflammatory effects, making it a candidate for treating conditions like arthritis and other inflammatory diseases.
- Drug Delivery Systems: Dihydroartemisinin can be incorporated into nanoparticles for targeted drug delivery, enhancing the efficacy and reducing side effects of treatments.
- Veterinary Medicine: Its use extends to veterinary applications, particularly in treating parasitic infections in livestock, contributing to better animal health and productivity.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.