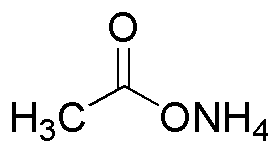
Ammonium acetate is widely utilized in research focused on:
- Buffer Solutions: It serves as a key component in biological and chemical buffer systems, helping to maintain pH stability in various experiments, particularly in biochemical assays.
- Food Industry: Used as a food additive, it acts as a flavoring agent and preservative, enhancing the taste and shelf-life of processed foods.
- Pharmaceutical Applications: It plays a role in drug formulation, aiding in the solubility and stability of active ingredients, which is crucial for effective medication delivery.
- Textile Industry: Employed in dyeing processes, it helps to improve the uptake of dyes in fabrics, resulting in more vibrant and long-lasting colors.
- Laboratory Reagent: Commonly used in various chemical reactions and syntheses, it is valued for its ability to act as a source of ammonium ions, facilitating numerous analytical procedures.
General Information
Properties
Safety and Regulations
Applications
Ammonium acetate is widely utilized in research focused on:
- Buffer Solutions: It serves as a key component in biological and chemical buffer systems, helping to maintain pH stability in various experiments, particularly in biochemical assays.
- Food Industry: Used as a food additive, it acts as a flavoring agent and preservative, enhancing the taste and shelf-life of processed foods.
- Pharmaceutical Applications: It plays a role in drug formulation, aiding in the solubility and stability of active ingredients, which is crucial for effective medication delivery.
- Textile Industry: Employed in dyeing processes, it helps to improve the uptake of dyes in fabrics, resulting in more vibrant and long-lasting colors.
- Laboratory Reagent: Commonly used in various chemical reactions and syntheses, it is valued for its ability to act as a source of ammonium ions, facilitating numerous analytical procedures.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Numéro de catalogue
Numéro de lot/série
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.