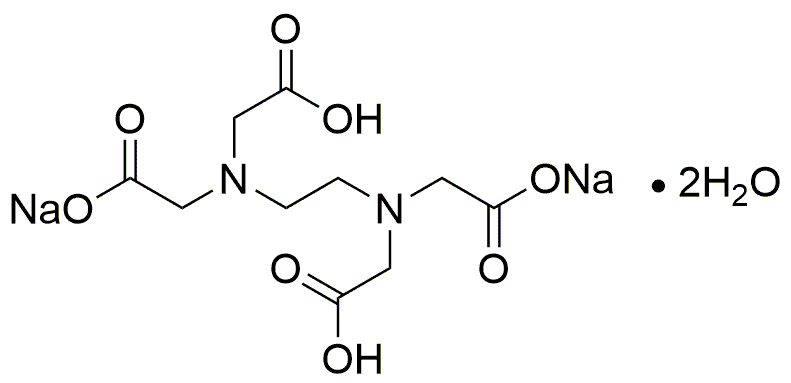
Ethylenediaminetetraacetic acid disodium salt, dihydrate is widely utilized in research focused on:
- Metal Ion Chelation: This compound effectively binds to metal ions, making it invaluable in various industries, including water treatment, where it helps remove harmful metals from wastewater.
- Pharmaceutical Applications: It is used in pharmaceuticals to stabilize formulations by sequestering metal ions that could catalyze degradation, enhancing the shelf life and efficacy of medications.
- Food Preservation: In the food industry, it acts as a preservative by preventing metal-catalyzed spoilage reactions, thus prolonging the freshness of products like canned foods and beverages.
- Laboratory Reagents: Researchers utilize it as a chelating agent in biochemical assays and experiments, improving accuracy by controlling metal ion concentrations in reactions.
- Cosmetic Formulations: Its ability to bind metal ions is also leveraged in cosmetics to prevent discoloration and degradation of active ingredients, ensuring product stability and safety.
General Information
Properties
Safety and Regulations
Applications
Ethylenediaminetetraacetic acid disodium salt, dihydrate is widely utilized in research focused on:
- Metal Ion Chelation: This compound effectively binds to metal ions, making it invaluable in various industries, including water treatment, where it helps remove harmful metals from wastewater.
- Pharmaceutical Applications: It is used in pharmaceuticals to stabilize formulations by sequestering metal ions that could catalyze degradation, enhancing the shelf life and efficacy of medications.
- Food Preservation: In the food industry, it acts as a preservative by preventing metal-catalyzed spoilage reactions, thus prolonging the freshness of products like canned foods and beverages.
- Laboratory Reagents: Researchers utilize it as a chelating agent in biochemical assays and experiments, improving accuracy by controlling metal ion concentrations in reactions.
- Cosmetic Formulations: Its ability to bind metal ions is also leveraged in cosmetics to prevent discoloration and degradation of active ingredients, ensuring product stability and safety.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Numéro de catalogue
Numéro de lot/série
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.