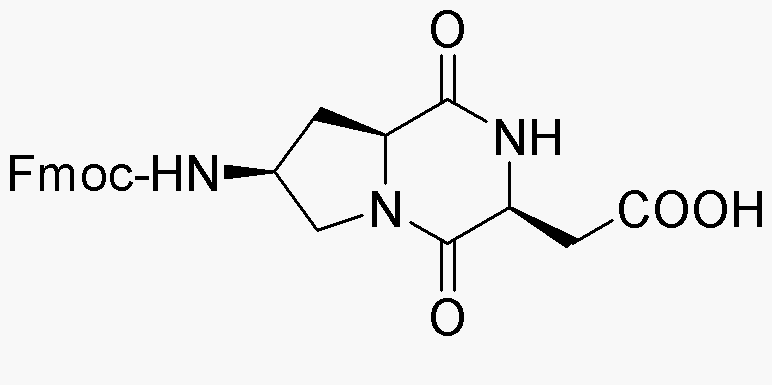
Fmoc-(2S,6S,9S)-6-amino-2-carboxymethyl-3,8-diazabicyclo-(4,3,0)-nonane-1,4-dione is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a protecting group in solid-phase peptide synthesis, allowing for the selective modification of amino acids without interfering with other functional groups.
- Drug Development: It is used in the design of peptide-based drugs, particularly in the development of therapeutics targeting specific diseases, enhancing bioavailability and efficacy.
- Bioconjugation: The chemical is valuable in bioconjugation techniques, facilitating the attachment of biomolecules to surfaces or other compounds, which is crucial in diagnostics and therapeutic applications.
- Research in Neuroscience: Its unique structure allows for the exploration of neuroactive peptides, aiding in the understanding of neurological pathways and potential treatments for neurological disorders.
- Custom Synthesis: Researchers utilize this compound for custom synthesis projects, benefiting from its versatility in creating novel compounds for various applications in pharmaceuticals and materials science.
General Information
Properties
Safety and Regulations
Applications
Fmoc-(2S,6S,9S)-6-amino-2-carboxymethyl-3,8-diazabicyclo-(4,3,0)-nonane-1,4-dione is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a protecting group in solid-phase peptide synthesis, allowing for the selective modification of amino acids without interfering with other functional groups.
- Drug Development: It is used in the design of peptide-based drugs, particularly in the development of therapeutics targeting specific diseases, enhancing bioavailability and efficacy.
- Bioconjugation: The chemical is valuable in bioconjugation techniques, facilitating the attachment of biomolecules to surfaces or other compounds, which is crucial in diagnostics and therapeutic applications.
- Research in Neuroscience: Its unique structure allows for the exploration of neuroactive peptides, aiding in the understanding of neurological pathways and potential treatments for neurological disorders.
- Custom Synthesis: Researchers utilize this compound for custom synthesis projects, benefiting from its versatility in creating novel compounds for various applications in pharmaceuticals and materials science.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.