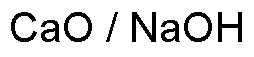Soda lime is widely utilized in research focused on various applications:
- Carbon Dioxide Absorption: Commonly used in anesthesia machines and breathing apparatus to absorb CO2, ensuring that patients receive fresh oxygen during procedures.
- Laboratory Applications: Employed in chemical laboratories for drying gases and removing moisture, which is crucial for accurate experimental results.
- Environmental Monitoring: Utilized in environmental studies to capture and analyze greenhouse gases, aiding in climate change research and policy-making.
- Industrial Processes: Applied in the production of various chemicals, where it serves as a reagent for neutralizing acids and controlling pH levels in chemical reactions.
- Waste Treatment: Used in the treatment of industrial waste, particularly for removing acidic components, thereby improving waste management practices.
General Information
Properties
Safety and Regulations
Applications
Soda lime is widely utilized in research focused on various applications:
- Carbon Dioxide Absorption: Commonly used in anesthesia machines and breathing apparatus to absorb CO2, ensuring that patients receive fresh oxygen during procedures.
- Laboratory Applications: Employed in chemical laboratories for drying gases and removing moisture, which is crucial for accurate experimental results.
- Environmental Monitoring: Utilized in environmental studies to capture and analyze greenhouse gases, aiding in climate change research and policy-making.
- Industrial Processes: Applied in the production of various chemicals, where it serves as a reagent for neutralizing acids and controlling pH levels in chemical reactions.
- Waste Treatment: Used in the treatment of industrial waste, particularly for removing acidic components, thereby improving waste management practices.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.