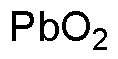Lead(IV) oxide is widely utilized in research focused on:
- Batteries: It is a key component in lead-acid batteries, providing high energy density and reliability, making it ideal for automotive and backup power applications.
- Ceramics: This compound is used in the production of ceramic glazes, enhancing durability and providing a glossy finish, which is essential in the pottery and tile industries.
- Electronics: Lead(IV) oxide serves as a semiconductor material in electronic devices, contributing to improved performance in various applications, including sensors and photovoltaic cells.
- Glass Manufacturing: It is employed in the production of specialty glass, such as optical glass, where it improves refractive index and clarity, making it valuable in optics and lens production.
- Research Applications: In laboratories, it is used as a reagent in chemical synthesis and as a catalyst, facilitating various reactions and contributing to advancements in materials science.
General Information
Properties
Safety and Regulations
Applications
Lead(IV) oxide is widely utilized in research focused on:
- Batteries: It is a key component in lead-acid batteries, providing high energy density and reliability, making it ideal for automotive and backup power applications.
- Ceramics: This compound is used in the production of ceramic glazes, enhancing durability and providing a glossy finish, which is essential in the pottery and tile industries.
- Electronics: Lead(IV) oxide serves as a semiconductor material in electronic devices, contributing to improved performance in various applications, including sensors and photovoltaic cells.
- Glass Manufacturing: It is employed in the production of specialty glass, such as optical glass, where it improves refractive index and clarity, making it valuable in optics and lens production.
- Research Applications: In laboratories, it is used as a reagent in chemical synthesis and as a catalyst, facilitating various reactions and contributing to advancements in materials science.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Número de catálogo
Número de lote/lote
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.