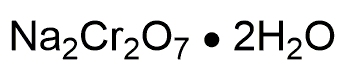Sodium dichromate dihydrate is widely utilized in research focused on:
- Metal Finishing: This compound is commonly used in the electroplating industry to provide a protective and decorative coating on metals, enhancing their corrosion resistance and aesthetic appeal.
- Analytical Chemistry: It serves as a reagent in various analytical procedures, particularly in the determination of the concentration of certain substances through titration methods.
- Textile Industry: Sodium dichromate dihydrate is employed in dyeing processes, particularly for fixing dyes on fabrics, which improves color fastness and vibrancy.
- Wood Preservation: This chemical is used in the treatment of wood to protect it from fungal decay and insect damage, significantly extending the lifespan of wooden structures.
- Laboratory Research: It is utilized in various laboratory experiments, including the synthesis of other chromium compounds, due to its strong oxidizing properties.
General Information
Properties
Safety and Regulations
Applications
Sodium dichromate dihydrate is widely utilized in research focused on:
- Metal Finishing: This compound is commonly used in the electroplating industry to provide a protective and decorative coating on metals, enhancing their corrosion resistance and aesthetic appeal.
- Analytical Chemistry: It serves as a reagent in various analytical procedures, particularly in the determination of the concentration of certain substances through titration methods.
- Textile Industry: Sodium dichromate dihydrate is employed in dyeing processes, particularly for fixing dyes on fabrics, which improves color fastness and vibrancy.
- Wood Preservation: This chemical is used in the treatment of wood to protect it from fungal decay and insect damage, significantly extending the lifespan of wooden structures.
- Laboratory Research: It is utilized in various laboratory experiments, including the synthesis of other chromium compounds, due to its strong oxidizing properties.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Número de catálogo
Número de lote/lote
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.